Blood Pressure Medications Recalled For Possible High Carcinogen Levels
Untreated heart failure increases the risk of hospitalization and death. Candida has a capsule and forms true hyphae and pseudohyphae.

Blood Pressure Drug Recalls An Update On Valsartan Society News Supchina
50 mg per 1X D 9212020 Email.
/cloudfront-us-east-1.images.arcpublishing.com/gray/MYC4NDVDSNFBPDGTVLYJEP43JA.png)
Blood pressure medications recalled for possible high carcinogen levels. Candida albicans is a common organism found in the oral cavity flora that causes candidiasis in certain clinical situations. Zantaca drug considered safe for decadesmay carry previously-undisclosed cancer risks from the carcinogen N-nitrosodimethylamine NDMA. High school collegeuniversity or professional and we will assign a writer who has a respective degree.
Possible Zantac Lawsuits for High Levels of NDMA. Nov 18 2019 Entresto is a prescription brand-name medication used to treat certain types. This expansion adds six lots of the 50 mg strength and four more lots of the 100 mg strength described asThe recall covers 25 mg 50 mg and 100 mg dosages.
The Fat Burning Kitchen The Top 101 Foods that Fight Aging The Diabetes Fix Type 2 diabetes is fast becoming a real epidemic in civilized countries. You can choose your academic level. Contact our law firm for a free consultation about Zantac cases class lawsuits and N Nitrosodimethylamine NDMA.
You can get up to 0 per call per phone line included in the settlement. Professional and Experienced Academic Writers. Many are native speakers and able to perform any task for which you.
While its still unknown how Zantac is contaminated with NDMA the finding has prompted numerous voluntary recalls as well as stores pulling the drug. I started the process which is the easiest process in the world by the way and I have to say I feel so much better. The agency determined that one additional cancer case would occur for every 8000 people who took the drug for four years.
Severe side effects from drugs can range from infections to death. Losartan is used to treat high blood pressure hypertension. The statistics show an ever-increasing trend of obesity diabetes and its related complications like heart disease kidney disease and peripheral neuropathy.
The current Philips CPAP lawsuits allege that the following sleep apnea devices. Our team may be able to help you if you took Zantac or generic ranitidine medications and see if it is causing cancers. Two kinds of blood pressure medication recalled for possibly too much of a carcinogen.
Legal advice may mean the difference for your cases. It adheres to mucosal surfaces and is capable of superficial mucosal invasion. After finding high levels of NDMA impurities.
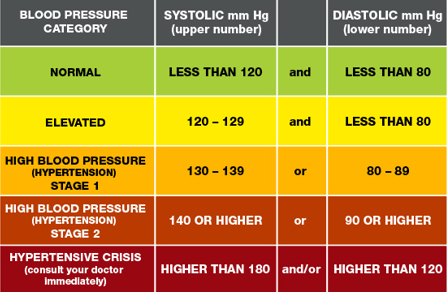
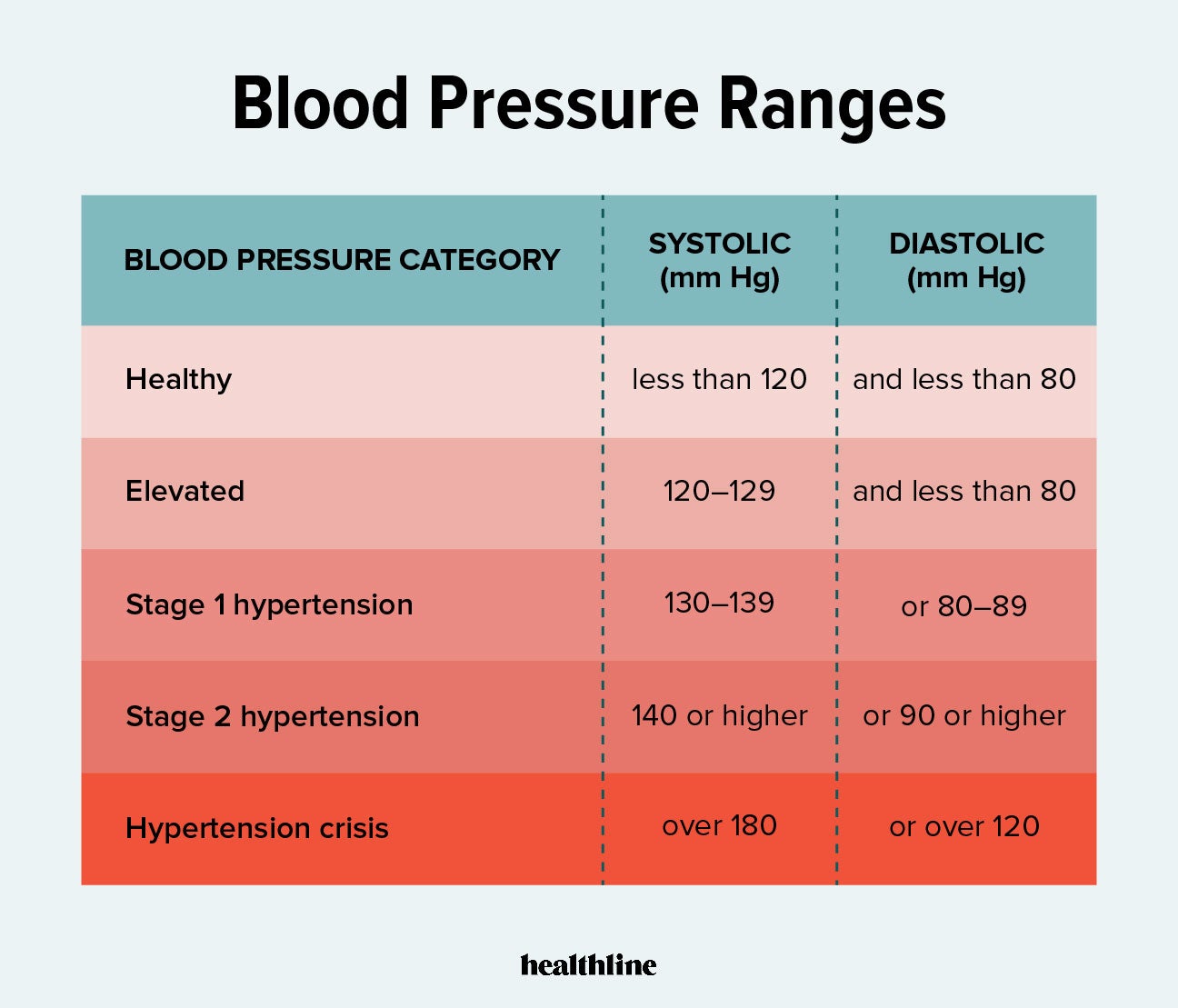
NDMA is found. Diabetes nutrition app e119. Untreated hypertension high blood pressure leads to an increase in the risk of heart attacks and stroke.
Two kinds of blood pressure medication recalled for possibly too much of a carcinogen Updated October 16 2021 608 PM Leak sheds light on family. Cat Ebeling co-author of the best-sellers. However the FDA has estimated the cancer risk for NDMA-contaminated valsartan a blood pressure drug.
High blood pressure is a common condition and when not treated it can cause damage to Oct 29 2018 On September 11 th James Jones a Missouri man filed an MDL class action lawsuit in the U. Recalled Zantac products exposed users to high levels of carcinogen. Just by my responses to his questions Robert was able to estimate my hormone levels.
Food and Drug Administration can add warnings to the drugs label including its stringent black box warningIn rare cases the drug is recalled or removed from the market. Sure enough after the full blood work came back he was on the money. Bi-Level Positive Airway Pressure Bi-Level PAPContinuous Positive Airway Pressure CPAP andMechanical Ventilators.
The FDAs data show NDMA at almost 10x the safe. This is the same contaminant that has been found in blood pressure medications such as Valsartan. We have a team of professional writers with experience in academic and business writing.
A probable carcinogen in certain drugs. My hair got so very thin it. All have a design defect that causes users to ingest or inhale degraded foam particlesThis is particularly true of the Philips DreamStation series.
For blood pressure medications specifically a new drug may make the patients blood pressure too high or too low and finding the right dose of a. Under chapter 362 Laws of 2019 if a child plans on attending or is attending a center early. Exempt or exemption means as applied to immunizations a type of immunization status where a child has not been fully immunized against one or more vaccine preventable diseases required by chapter 246-105 WAC for full immunization due to medical religious philosophical or personal reasons.
Prolonged use of these devices may lead to. If a drug has numerous reports of a particular problem the US.
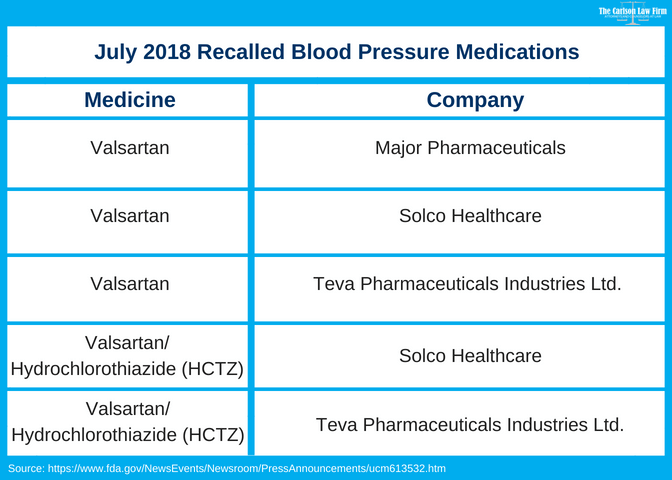
Valsartan Recall Fda Finds Carcinogenic Impurity In Blood Pressure Meds

Some Blood Pressure Drugs Are Linked With Better Memory Harvard Health

Recall Of Blood Pressure Medication Losartan Expands Over Cancer Concerns Everyday Health

Valsartan Another Cancer Causing Chemical Found In Widely Used Blood Pressure Medication Cbs News
The Role Of Gut Bacteria In Blood Pressure
Blood Pressure Medication Recall How It Happened What You Should Do

Hsa Recalls 3 Brands Of High Blood Pressure Drug Over Cancer Risk Today

Valsartan Recall 4 Things Patients Should Know Cnn

Blood Pressure Medication Recalled For Cancer Risk
High Blood Pressure Drug Recall Expands Again Over Cancer Risk Slashgear
/GettyImages-1185738318-c4f36650f4e14501b55dabd290c98abe.jpg)
Losartan Uses Side Effects Dosages Precautions
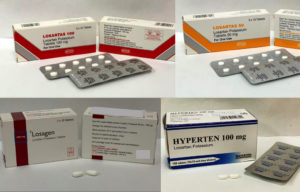
3 Brands Of High Blood Pressure Medication Losartan Recalled For High Levels Of Cancer Causing Contaminant Dr Siew Com

/cloudfront-us-east-1.images.arcpublishing.com/gray/IFRWTBMGXFAAVL36BTAQD77TQ4.png)
/cloudfront-us-east-1.images.arcpublishing.com/gray/A7A5CIT5GREO7OVZPSG4ZHG2U4.png)
